Chemistry Lesson 01: Particle Theory of Matter
Short Summary:
This chemistry lesson introduces the Particle Theory of Matter, explaining that all matter is made up of tiny particles in constant motion. The theory is used to explain the differences between solids, liquids, and gases, and to classify matter as pure substances (elements and compounds) or mixtures (homogeneous and heterogeneous). Examples like perfume diffusing in a room, ice melting into water, and the composition of steel and saltwater illustrate the concepts. The lesson uses animations to visually represent particle behavior and concludes with a classification game to reinforce the understanding of elements, compounds, and mixtures. The implications are a foundational understanding of chemistry, enabling the explanation of various material properties and behaviors.
Detailed Summary:
The lesson is divided into several sections:
1. Introduction to the Particle Theory: The lesson begins by introducing the Particle Theory as the fundamental basis of chemistry, explaining that it involves understanding the movement and interaction of tiny particles of matter. The example of perfume diffusing in a room is used to illustrate how particles move and interact. The speaker emphasizes that this theory replaces the ancient Greek concept of four elements (earth, air, fire, water) as a more accurate model.
2. Particle Behavior and States of Matter: This section uses an animation to show how particles are in constant random motion, bouncing off each other and their containers. The animation demonstrates how changes in temperature affect particle speed, and how the arrangement of particles determines the state of matter (solid, liquid, gas). The example of water transitioning through its three states is shown.
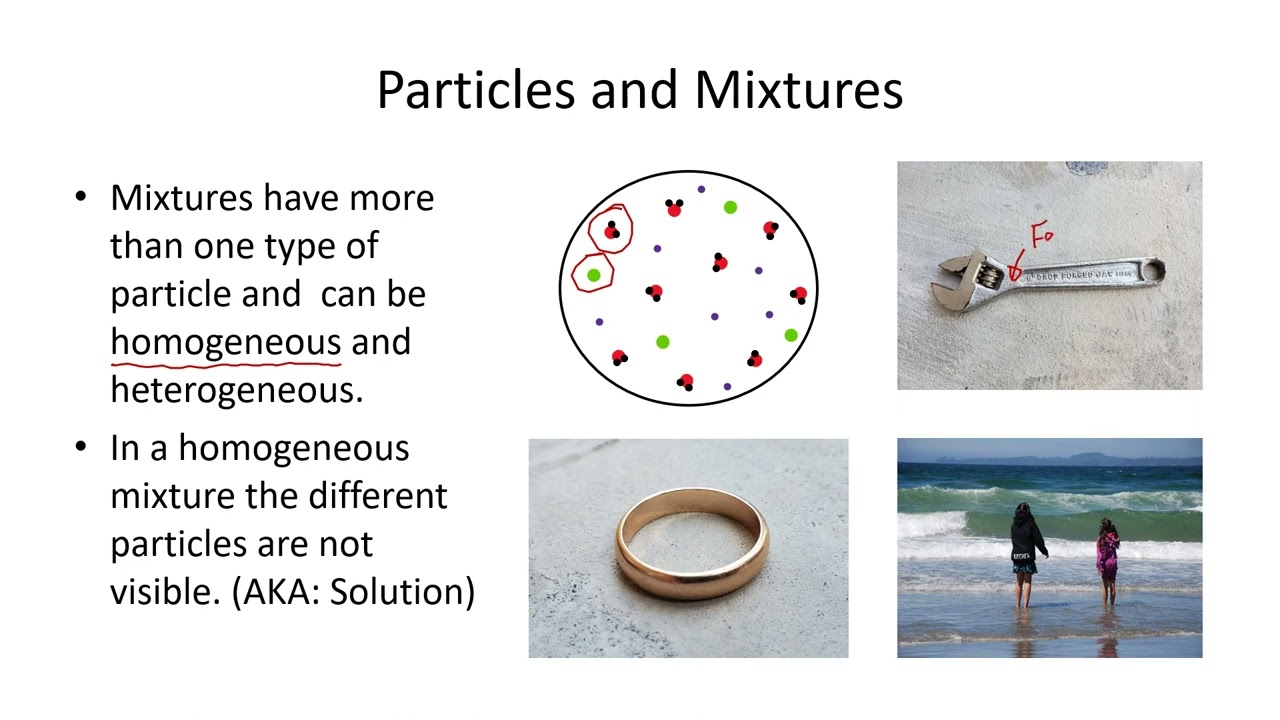
3. Classification of Matter: The lesson then moves on to classifying matter. It defines pure substances as having only one type of particle (either elements or compounds) and mixtures as having more than one type of particle.
4. Pure Substances: Elements and Compounds: Elements are defined as pure substances with all particles being the same type of atom (e.g., carbon, oxygen). Compounds are defined as pure substances where different types of atoms are bonded together (e.g., water (H₂O), table salt (NaCl)). The difference between molecular and ionic compounds is briefly explained.
5. Mixtures: Homogeneous and Heterogeneous: Mixtures are classified as homogeneous (where different particles are indistinguishable, like saltwater or steel) and heterogeneous (where different particles are visible, like granola or milk). Examples of each type are provided, including discussion of solutions, suspensions, and emulsions.
6. Classification Game and Summary: A game is played to test understanding by identifying elements, compounds, and mixtures based on visual representations of particles. The lesson concludes with a summary diagram organizing the classification of matter from the broadest category (matter) down to specific types (elements, compounds, homogeneous mixtures, heterogeneous mixtures). The speaker reinforces the key idea that all matter can be understood through the lens of its constituent particles and their interactions.