How To Calculate The Molar Mass of a Compound - Quick & Easy!
Short Summary:
This video explains how to calculate the molar mass of a compound. It starts with basic examples using elemental atoms (nitrogen, fluorine) and progresses to more complex molecules (ozone, sulfur, carbon dioxide, silicon tetrafluoride, glucose). The video then demonstrates calculating molar mass for ionic compounds, requiring the determination of the chemical formula first (calcium phosphate, vanadium(V) hydrogen phosphate). The key method involves finding the atomic mass of each element from the periodic table, multiplying by the number of atoms of that element in the compound, and summing the results to obtain the molar mass in grams per mole. The applications are widespread in chemistry, particularly in stoichiometry and other quantitative chemical calculations.
Detailed Summary:
The video is structured in several sections, progressively increasing the complexity of the compounds used for demonstration:
Section 1: Introduction to Molar Mass: The video begins by defining molar mass and its relationship to mass and moles. It uses elemental nitrogen (N) and fluorine (F) as simple examples, showing how their atomic masses from the periodic table directly represent their molar masses (14.01 g/mol for N and 19 g/mol for F).
Section 2: Molar Mass of Simple Molecules: This section expands to calculating the molar mass of ozone (O3) and elemental sulfur (S8). The process is demonstrated: find the atomic mass of each element, multiply by the number of atoms in the molecule, and sum the results (48 g/mol for O3 and 256.56 g/mol for S8).
Section 3: Molar Mass of More Complex Molecules: The video then tackles more complex molecules like carbon dioxide (CO2) and silicon tetrafluoride (SiF4). The same method is applied, showing the step-by-step calculation (44.01 g/mol for CO2 and 104.09 g/mol for SiF4). The instructor encourages viewers to pause and try these examples themselves.
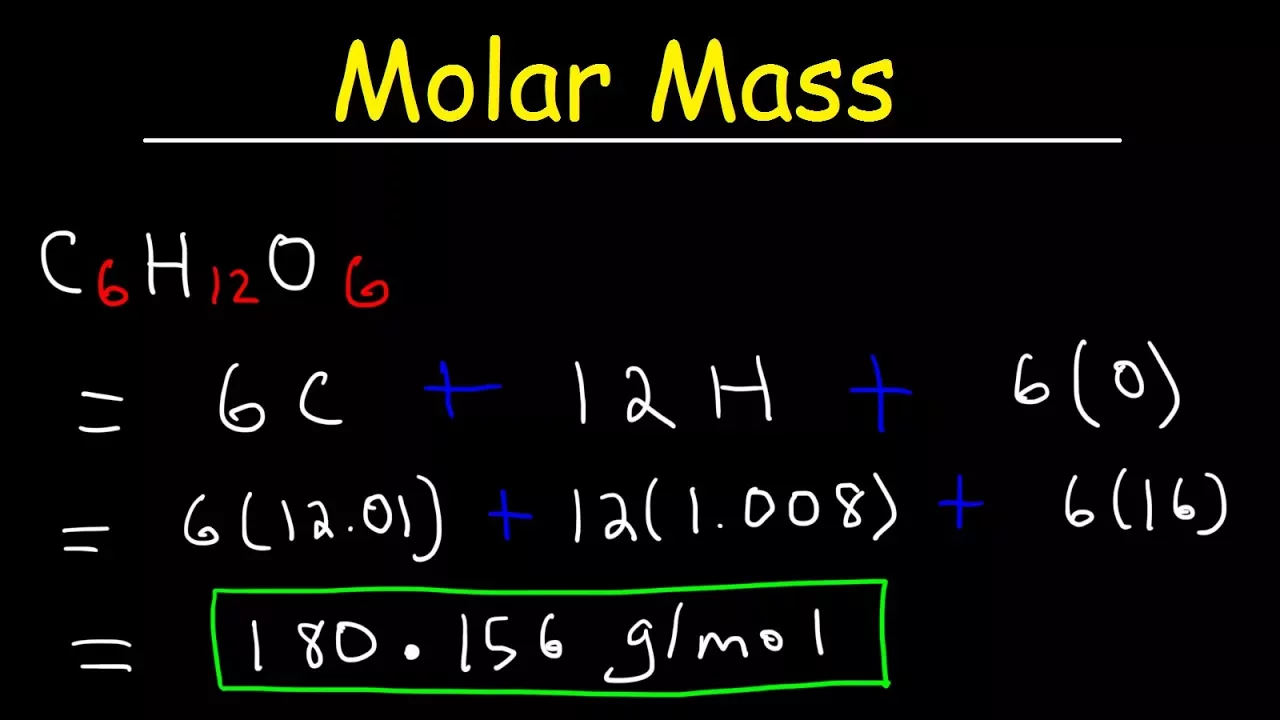
Section 4: Molar Mass of Glucose: This section focuses on calculating the molar mass of glucose (C6H12O6). The instructor demonstrates a systematic approach, separating the calculation for each element (carbon, hydrogen, oxygen) before summing the results (180.16 g/mol for glucose).
Section 5: Molar Mass of Ionic Compounds: The final sections deal with ionic compounds, emphasizing the need to first determine the correct chemical formula. The examples include calcium phosphate (Ca3(PO4)2) and vanadium(V) hydrogen phosphate. The instructor explains how to determine the chemical formula based on the charges of the ions involved. The calculation of molar mass then follows the same principle as before, but with more elements to consider (310.18 g/mol for Ca3(PO4)2 and 581.77 g/mol for V2(HPO4)5). The video highlights the importance of understanding ionic compound nomenclature and formula writing. No specific quotes are emphasized, but the consistent message is the straightforward application of the core method.