Link to original video by Rachel's Biology Videos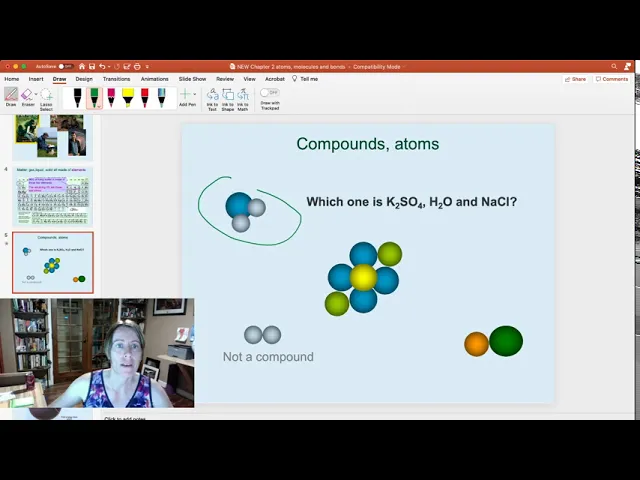
Compounds and chemical formulae

Summary of "Compounds and Chemical Formulae"
Short Summary:
This video introduces the concept of compounds, which are substances formed by combining different types of atoms. It explains that chemical formulae are shorthand representations of the composition of compounds, indicating the types and quantities of atoms needed to create them. The video uses examples like K2SO4, H2O, and NaCl to illustrate how to interpret chemical formulae and match them to visual representations of compounds.
Detailed Summary:
Section 1: Introduction to Elements and Atoms
- The video begins by briefly reviewing elements as the fundamental building blocks of the universe.
- It emphasizes that elements exist as atoms, which are incredibly small particles.
- The speaker uses the analogy of Lego blocks to help visualize the concept of atoms.
- While acknowledging that atoms are not simply round balls, the video simplifies them as such for the sake of understanding in a biological context.
Section 2: Compounds and Their Formation
- The video defines compounds as substances formed by combining different types of atoms.
- It uses diagrams with colored balls representing different atoms to illustrate the formation of compounds.
- The speaker emphasizes that a compound must contain at least two different types of atoms.
Section 3: Chemical Formulae as Shorthand Representations
- The video introduces chemical formulae as a concise way to represent the composition of compounds.
- It explains that chemical formulae indicate the types and quantities of atoms needed to create a specific compound.
- The speaker uses examples like K2SO4, H2O, and NaCl to demonstrate how to interpret chemical formulae.
- He explains that the numbers in a formula refer to the number of atoms of the element preceding them.
- The speaker also clarifies that a number of 1 is implied if there is no number written after an element symbol.
Section 4: Matching Formulae to Diagrams
- The video encourages viewers to practice matching chemical formulae with the corresponding diagrams of compounds.
- This exercise reinforces the understanding of how to interpret chemical formulae and identify the constituent atoms in a compound.
Notable Quotes:
- "Elements are those basic building blocks of the universe."
- "Atoms are incredibly tiny... you probably can't even wrap your head around how tiny they even are."
- "To build a cell or a living thing, we're going to take a whole bunch of atoms and we have to join them together."
- "A chemical formula is really a shorthand way of writing a recipe for which how many of which kind of atoms do you need to make this substance."